Abstract
Background
Despite recent advances in treatments for patients with chronic lymphocytic leukemia (CLL), such as Bruton's tyrosine kinase (BTK) inhibitors and a Bcl-2 inhibitor, the disease remains generally incurable (Todorovic Z et al. Curr Oncol. 2022). Brexucabtagene autoleucel (brexu-cel; KTE-X19) is a CD19-directed genetically modified autologous T-cell (CAR T-cell) immunotherapy approved for use in patients with relapsed/refractory (R/R) mantle cell lymphoma and in patients with R/R B-cell precursor acute lymphoblastic leukemia; however, no CAR T-cell therapies are currently approved in CLL. The multicohort, multicenter Phase 1 ZUMA-8 (NCT03624036) trial is the first to evaluate the safety and tolerability of KTE-X19 in patients with R/R CLL.
Methods
In ZUMA-8, patients had R/R CLL after treatment with ≥2 prior lines of therapy (including a BTK inhibitor). Leukapheresis was performed within ~5 days after confirmed eligibility. Optional bridging therapy (continuation of preceding targeted therapy, anti-CD20 antibody, and/or high-dose corticosteroids) before conditioning therapy (CC) was allowed. Patients received 3 days of CC (fludarabine 30 mg/m2/day and cyclophosphamide 500 mg/m2/day) before KTE-X19 infusion. Patients in Cohorts 1 and 2 were administered 1 × 106 and 2 × 106 anti-CD19 CAR T cells/kg, respectively. Patients in Cohort 3 (patients who had low tumor burden, defined as ≤1% malignant cells in peripheral blood or absolute lymphocyte count [ALC] <5,000 cells/µL [patients with small lymphocytic lymphoma were also allowed]) and Cohort 4 (patients with any degree of tumor burden who were treated with the BTK inhibitor ibrutinib [alone or in combination] as the last line of therapy up to 30 hours prior to leukapheresis) received a target dose of 1 × 106 anti-CD19 CAR T cells/kg. Patients were hospitalized for observation ≥7 days after infusion. The primary endpoint was the incidence of dose-limiting toxicities (DLTs). Secondary endpoints were incidence of adverse events (AEs), objective response rate per investigator review according to the International Workshop on Chronic Lymphocytic Leukemia 2018 criteria, and CAR T-cell expansion.
Results
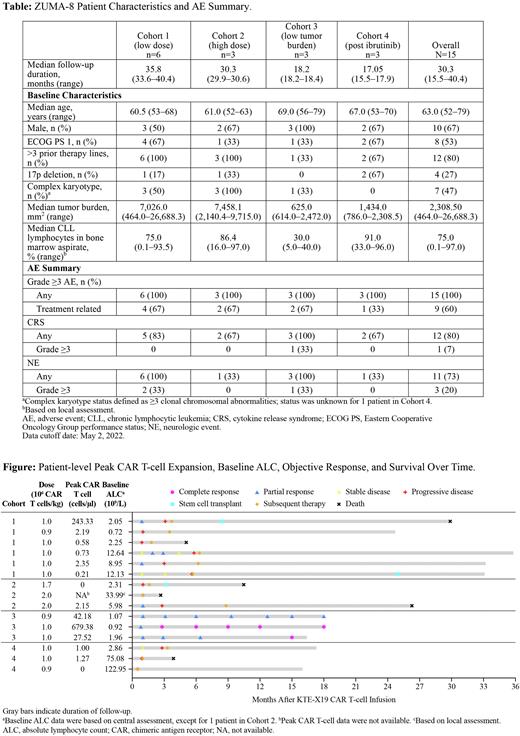
A total of 15 patients received KTE-X19 therapy across Cohort 1 (n=6), Cohort 2 (n=3), Cohort 3 (n=3, all with CLL), and Cohort 4 (n=3). At the data cutoff date of May 2, 2022, the median follow-up duration was 30.3 months (range, 15.5-40.4 months; Table). The median age was 63.0 years (range, 52-79 years), 10 patients (67%) were male, 8 patients (53%) had an Eastern Cooperative Oncology Group performance status of 1, 4 patients (27%) had a 17p deletion, and 7 patients (47%) presented with a complex karyotype (defined as ≥3 clonal chromosomal abnormalities). Patients were heavily pretreated; 12 patients (80%) received >3 prior lines of therapy, and 13 of 15 patients received bridging therapy. DLTs were observed in 1 patient in Cohort 3 (Grade 3-4 hypocalcemia, hyponatremia, hypotension, and cytokine release syndrome [CRS] events that met prespecified criteria for DLTs). Grade ≥3 AEs and Grade ≥3 serious AEs were reported in all patients (100%) and 5 patients (33%), respectively. Grade ≥3 treatment-related AEs were reported in 9 patients (60%). In addition to Grade 4 CRS reported in 1 patient (7%), Grade ≥3 neurologic events were reported in 3 patients (20%). Excluding disease progression, there were no Grade 5 AEs. As of the data cutoff date, objective responses were observed in 7 of 15 patients, including 2 patients with complete responses (CR; Figure). Two of 3 patients with low tumor burden (Cohort 3) achieved CR and 1 patient achieved a partial response. Appreciable CAR T-cell expansion occurred in 4 of 15 patients overall and in 3 of 3 patients with a low tumor burden. Peak CAR T-cell expansion (range, 0-679.38 cells/µL; n=14) had an apparent weak inverse correlation with baseline ALC (range, 0.72-122.95 × 109/L; n=15). Additional translational data will be presented.
Conclusions
KTE-X19 therapy did not have any new safety signals in patients with R/R CLL. Peak CAR T-cell expansion and objective responses in heavily pretreated patients with low tumor burden appeared to be improved compared with other cohorts.
Disclosures
Davids:AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol-Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees; BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Ascentage Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Merck: Consultancy; Ono Pharmaceuticals: Consultancy; Novartis: Research Funding; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel expenses, Research Funding; Eli Lilly and Company: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy; TG Therapeutics: Consultancy, Research Funding; Research to Practice: Honoraria; Adaptive Biotechnologies: Consultancy, Membership on an entity's Board of Directors or advisory committees; Verastem: Consultancy, Research Funding. Kenderian:Novartis: Consultancy, Patents & Royalties: CART cell therapy , Research Funding, Speakers Bureau; Kite/Gilead: Consultancy, Research Funding, Speakers Bureau; Juno/BMS: Consultancy, Research Funding, Speakers Bureau; Humanigen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties: CART cell therapy , Research Funding, Speakers Bureau; Lentigen: Research Funding; Tolero: Research Funding; Viracta/Sunesis: Research Funding; LEAH Labs: Current holder of stock options in a privately-held company, Research Funding; Morphosys: Research Funding; Life Engine: Current holder of stock options in a privately-held company; MustangBio: Patents & Royalties; Mettaforge: Patents & Royalties. Flinn:Constellation Pharmaceuticals: Research Funding; IGM Biosciences: Research Funding; Fate Therapeutics: Research Funding; Forty Seven: Research Funding; Tessa Therapeutics: Research Funding; Celgene: Research Funding; ArQule: Research Funding; Agios: Research Funding; Acerta Pharma: Research Funding; Unum Therapeutics: Research Funding; TG Therapeutics: Consultancy, Research Funding; Takeda: Consultancy; Servier Pharmaceuticals: Consultancy; Roche: Consultancy, Research Funding; Seattle Genetics: Research Funding; Nurix Therapeutics: Consultancy, Research Funding; Kite Pharma: Consultancy, Research Funding; Genmab: Consultancy; Genentech: Consultancy, Research Funding; Century Therapeutics: Consultancy; BeiGene: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; Pfizer: Research Funding; Merck: Research Funding; Loxo@Lilly: Research Funding; Forma Therapeutics: Research Funding; Biopath: Research Funding; Xencor: Consultancy; Bristol Myers Squibb: Research Funding; Gilead Sciences: Research Funding; Iksuda Therapeutics: Consultancy; Hutchison MediPharma: Consultancy; Janssen: Consultancy, Research Funding; InnoCare Pharma: Consultancy, Research Funding; CTI Biopharma: Research Funding; CALGB: Research Funding; City of Hope National Medical Center: Research Funding; Millenium Pharmaceuticals: Research Funding; Triphase Research & Development Corp: Research Funding; TCR2 Therapeutics: Research Funding; 2seventy bio: Research Funding; Pharmacyclics: Consultancy, Research Funding; Verastem: Consultancy, Research Funding; Vincerx Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Portola Pharmaceuticals: Research Funding; Infinity Pharmaceuticals: Research Funding; Incyte: Research Funding; Curis: Research Funding; Epizyme: Research Funding; Abbvie: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; MorphoSys: Consultancy, Research Funding; Secura Bio: Consultancy; Myeloid Therapeutics: Research Funding; Rhizen Pharmaceuticals: Research Funding; Trillium Therapeutics: Research Funding; CALIBR: Research Funding. Hill:Kite, a Gilead Company: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding. Ghia:Roche: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; BeiGene: Consultancy, Honoraria; MSD: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria; Lilly/Loxo: Consultancy, Honoraria. Byrne:Celularity, Concert: Consultancy, Other: DSMC; CTI: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Taiho: Research Funding; Karyopharm: Research Funding. Bartlett:Autolus, Bristol-Meyers Squibb, Celgene, Forty Seven, Janssen, Kite Pharma, Merck, Millennium, Pharmacyclics: Research Funding; Washington University School of Medicine: Current Employment; ADC Therapeutics, Roche/Genentech, Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding. Pagel:AsraZeneca: Consultancy; Kite/Gilead: Consultancy; Morphosys/Incyte: Consultancy; Epizyme: Consultancy; Actinium: Consultancy. Zheng:Kite, a Gilead Company: Current Employment; Gilead Sciences: Current holder of stock options in a privately-held company. Kanska:Kite, a Gilead company: Current Employment. Zhang:Kite, a Gilead company: Current Employment. Masouleh:Kite, a Gilead Company: Current Employment, Current equity holder in publicly-traded company, Other: Travel support; Lava: Current equity holder in publicly-traded company. Granados:Kite, a Gilead company: Current Employment. Pinilla Ibarz:AbbVie: Consultancy; SecuraBio: Research Funding; Pharmacyclics: Consultancy; AstraZeneca: Consultancy; Janssen Pharmaceuticals: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal